The first and only approved treatment
for non-segmental vitiligo repigmentation*1,2

Vitiligo’s
effect is not
just skin
deep5-8


Vitiligo
incidence,
prevalence and
demographics
Around 65-100 million people around the world are affected by vitiligo with a prevalence of around 0.4-1.6%.11-14 Non-segmental vitiligo accounts for 84-95% of vitiligo cases.15 Vitiligo is often misdiagnosed or under-reported, leading to an underestimated prevalence. According to a global survey, almost half of patients with vitiligo were previously misdiagnosed.16
Vitiligo affects men and women equally, although prevalence is slightly higher in females. Most patients will experience the onset of vitiligo by the age of 30 and around 70% of diagnosed patients will have facial involvement.14,17-21
‘less noticeble’
vitiligo with Opzelura®3,4



Opzelura® cream is convenient to apply
Opzelura® cream is applied twice a day to the depigmented skin areas up to a maximum of 10% of the body surface area (BSA).1
Opzelura® must be applied with at least 8 hours between
applications. Patients should avoid washing the treated skin
area for at least 2 hours after applying Opzelura®.1
To facilitate precise dosing, the ”fingertip unit” can be used.
A fingertip amount of cream can cover an area of skin the size of two palms.26,27
It’s important to remember vitiligo treatments work gradually. It usually takes 6–12 months to see visible repigmentation with Opzelura®, and results vary between patients.1
Opzelura® is generally well tolerated^
The most common adverse reaction is application site acne (5.8%). In the TruE-V1 and TRuE-V2 studies, no serious adverse events was considered to be related to Opzelura®.3
Opzelura® is contraindicated during pregnancy and breastfeeding.1

Your patients, vitiligo and Opzelura®
Effective vitiligo management often extends beyond medication alone.22 Consider having conversations with your vitiligo patients to discuss current available vitiligo treatments, and the psychosocial impact that vitiligo may be having on their lives.
The first approved treatment for non segmental vitiligo1,2
Opzelura®, which contains the active substance ruxolitinib, is the
first approved topical JAK1+2 inhibitor for vitiligo and has been approved for use in Europe.1,2
Reducing depigmentation
by selectively binding to JAK1 and JAK21
Inducing
repigmentation
melanocytes migrate from hair follicles and lesion edges by selectively binding to JAK1 and JAK2, reducing melanocyte destruction23,24
Preventing
relapses
Blocking JAK1 interrupts IL-15 signaling and reduces CD8+ T cell activity hypothesised to prevent relapse24,25
Q&A
- How big is the tube and how long does it last?
The tube size of Opzelura® is 100 grams. How long it lasts depends on the area of skin treated and the frequency of use. Patients should use it twice daily applying an appropriate amount of creme. Most patients have to use it for at least 6 months to see significant results. It’s important to remember vitiligo treatments work gradually.1The tube should only be used for 6 months after opening. - On which body parts does it work best?
Opzelura® can be used on most areas of the body, but the face area respond often better on treatment than other body parts.3 Opzelura® can also be applied to hands, arms, legs and others affected areas up to a maximum of 10 % (one tenth) of the total body surface area. This surface area represents the equivalent to ten times the palm of one hand with five fingers1. Opzelura® must not be used on the lips, the eyes, the mouth or vagina.1 - What type of vitiligo does it work for?
Opzelura® is effective for non-segmental vitiligo with facial involvement1. Non-segmental vitiligo is the most common type and causes depigmentation on both sides of the body in a symmetrical pattern15 Opzelura® has shown promising results in promoting repigmentation in non-segmental vitiligo.3 - How does the cream itself work – what makes it able to improve vitiligo?
The active ingredient, ruxolitinib, is a JAK inhibitor1. Vitiligo is believed to involve a dynamic interplay between several factors, including environmental triggers, immune system dysregulation and genetic susceptibility, leading to melanocyte (pigment cell) destruction.11,28,29 Ruxolitinib blocks certain pathways (JAK-STAT) that contribute to the immune response, allowing melanocytes to potentially regenerate and repopulate affected areas.30 - For which people does the treatment work – with a lot or a little vitiligo?
Opzelura® can be used for different severities of vitiligo, but should only be applied to affected areas up to 10 % of the total body surface area (BSA).1 - What ages can use the cream?
Opzelura® is approved for use in adults and children aged 12 and older for treating vitiligo.1 - Does it matter how long you have had vitiligo?
No, Opzelura® can be used regardless of how long time you have had vitiligo.1,3 - How is the cream applied? Amount?
Opzelura® should be applied twice daily with eight hours between applications. The amount depends on the size of the area being treated. Patients should apply a thin layer on the affected skin covering it fully but not over-applying.1 To facilitate accurate dosing, the “fingertip-unit” can be used. With the amount of one fingertip-unit of creme, a skin area of the size of 2 hand palms can be covered.26,27
After applying Opzelura®, patients should wait at least 2 hours before washing the treated skin area, using any other medicine, sunscreen, skin creams/oils or make-up on the same skin area. Patients should always apply sunscreen before staying in the sun as the natural sun protection is worse on the areas where the skin lacks pigment.1 It’s important to wash hands after application (unless the hands are treated).1 Opzelura® dries within 10 minutes after application, leaves no residue on the skin and therefore does not discolour clothes.
- Can vitiligo be cured by the treatment/can vitiligo disappear completely be the treatment?
Opzelura® does not cure vitiligo, but it has shown to significantly improve repigmentation in many cases.1 Opzelura® does not cure vitiligo but with continued use, patients often see substantial repigmentation in the treated areas. Continuous maintenance treatment may be necessary to sustain results.1
Side effects and contraindications
References
JAK, Janus Kinase; F-VASI, facial Vitiligo Area Scoring Index; T-VASI, total Vitiligo Area Scoring Index
1. Opzelura (ruxolitinib) 15 mg/g cream Summary of Product Characteristics; Date of revision 2025-07-03. Incyte Biosciences Distribution B.V. 2. Vitiligo International Patient Organisations Committee (Vipoc): Incyte Announces European Commission Approval Of Opzelura® (Ruxolitinib) Cream For The Treatment Of Non-Segmental Vitiligo With Facial Involvement In Adults And Adolescents (22-04-2023). Available at: https://www.vipoc.org/incyte-announces-european-commission-approval-of-opzelura-ruxolitinib-cream-for-the-treatment-of-non-segmental-vitiligo-with-facial-involvement-in-adults-and-adolescents/ (Accessed 21-01-2025). 3. Rosmarin D, Passeron T, PandyaAG, et al. Two Phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387(16):1445–1455.4. Batchelor JM, Tan W, Tour S, et al. Validation of the Vitiligo Noticeability Scale: a patient reported outcome measure of vitiligo treatment success. Br J Dermatol. 2016;174(2):386–394. 5. Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatol Basel Switz. 2020;236(6):571–592. 6. Krüger C, Schallreuter KU. Stigmatisation, avoidance behaviour and difficulties in coping are common among adult patients with vitiligo. Acta Derm Venereol. 2015;95(5):553–558. 7. Bibeau K, Hamzavi I, Ezzedine K. Mental health and psychosocial burden among patients living with vitiligo: findings from the global VALIANT study. Presented at Maui Derm for Dermatologists. 2022. 8. Phan K, Shumack S, Gupta M. Association between vitiligo and risk of suicide and suicidal ideation: systematic review and meta-analysis. Pigment Int. 2022;9(2):127. 9. Hadi A, et al. J Am Acad Dermatol. 2020;82(3):628–633. 10. Gill L, et al. J Am Acad Dermatol. 2016;74(2):295–302. 11. Bergqvist C, et al. J Dermatol. 2021;48:252–270. 12. VR Foundation. About Vitiligo. Available at: https://vrfoundation.org/ about_vitiligo (Accessed November 2024). 13. Krüger C, et al. Int J Dermatol. 2012;51:1206–1212. 14. Bibeau K, et al. J Eur Acad Dermatol Venereol. 2022;36:1831–1844. 15. Picardo M, et al. Nat Rev Dis Primers. 2015;1:15011 16. Bibeau K, et al. Presented at: Maui Derm for Dermatologists, 24 January 2022, Maui, HI. 17. Krüger C, et al. Int J Dermatol. 2012;51:1206–1212. 18. Talsania N, et al. Clin Exp Dermatol. 2010;35:736–739. 19. Gandhi K, et al. JAMA Dermatol. 2022;158:43–50. 20. Patel R, et al. Dermatol. 2023;239:227–234. 21. Ezzedine K, et al. Pigment Cell Melanoma Res. 2014;27:134–139. 22. Anbar T, et al. Exp Dermatol. 2014;23(4):219–223. 23. Birlea SA, Goldstein NB, Norris DA. Repigmentation through Melanocyte Regeneration in Vitiligo. Dermatol Clin. 2017;35(2):205–218. 24. Chen X, Guo W; Chang Y al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8+ T cells activation via JAK-STAT pathway in vitiligo. Free Radic Biol Med. 2019;139:80–91 25. Nolz JC, Richer MJ. Control of memory CD8+ T cell longevity and effector functions by IL-15. Mol Immunol. 2020;117:180-188. 26. Bewley A; Dermatology Working Group. Expert consensus:
time for a change in the way we advise our patients to use topical corticosteroids. Br J Dermatol. 2008 May;158(5):917-20. doi: 10.1111/j.1365-2133.2008.08479.x. Epub 2008 Feb 22. PMID: 18294314. 27. Long CC, Finlay AY. The finger-tip unit–a new practical measure. Clin Exp Dermatol. 1991 Nov;16(6):444-7. doi: 10.1111/j.1365-2230.1991. tb01232.x. PMID: 1806320. 28. Said‑Fernandez SL, Sanchez-Domínguez CN, Salinas-Santander MA, et al. Novel immunological and genetic factors associated with vitiligo: A review. Exp Ther Med. 2021;21(4):312. 29. Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257–265. 30. Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol. 2016 Dec;43:81-88.
^The only AE reported in the SmPC is application site acne.1
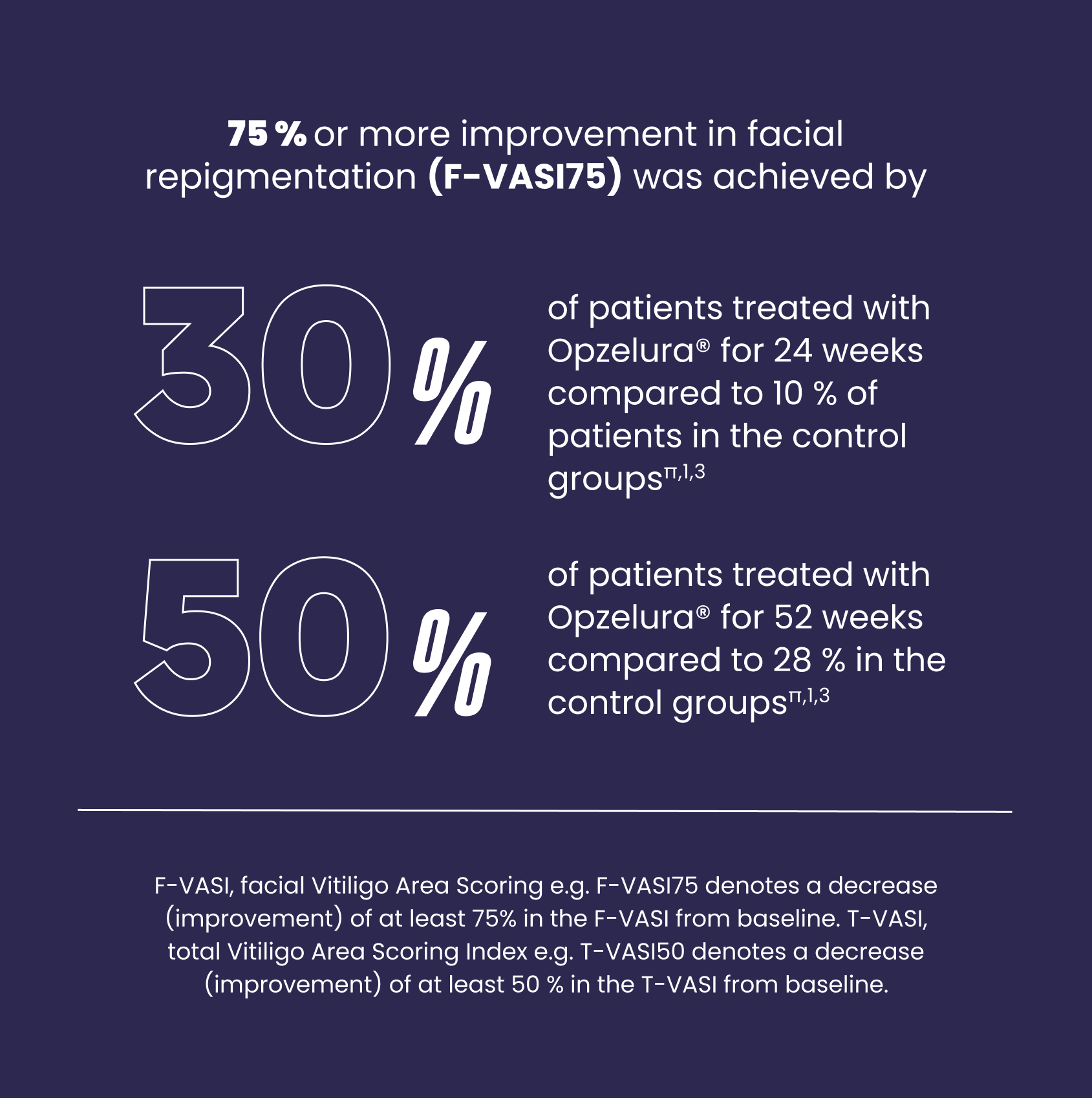
π After 24 weeks: The percentages are the proportion of patients who achieved F-VASI75 over the 24-week treatment period in pooled data from study TRuE-V1 and TRuE-V2 as shown in Figure 1 of the SmPC. TRuE-V1: N=221/330 patients; Response rate difference between test and control (95% CI)= 22.3 (14.214 to 30.471) p<0.0001. TruE-V2: N= 222/331 patients; Response rate difference between test and control (95% CI)= 19.5 (10.537 to 28.420) p<0.001. The control groups received vehicle cream without the active ingredient. After 52 weeks: The percentages are the proportion of patients who achieved F-VASI75 over the 52-week treatment period in pooled data from study TRuE-V1 and TRuE-V2 as shown in Figure 1 of the SmPC. The control group received vehicle cream without active ingredient in the first 24 weeks and then treatment with ruxolitinib cream in the last 28 weeks; TRuE-V1: N= 91/173 patients (52.6%) TRuE-V2: N 85/177 patients (48.0%).1,3
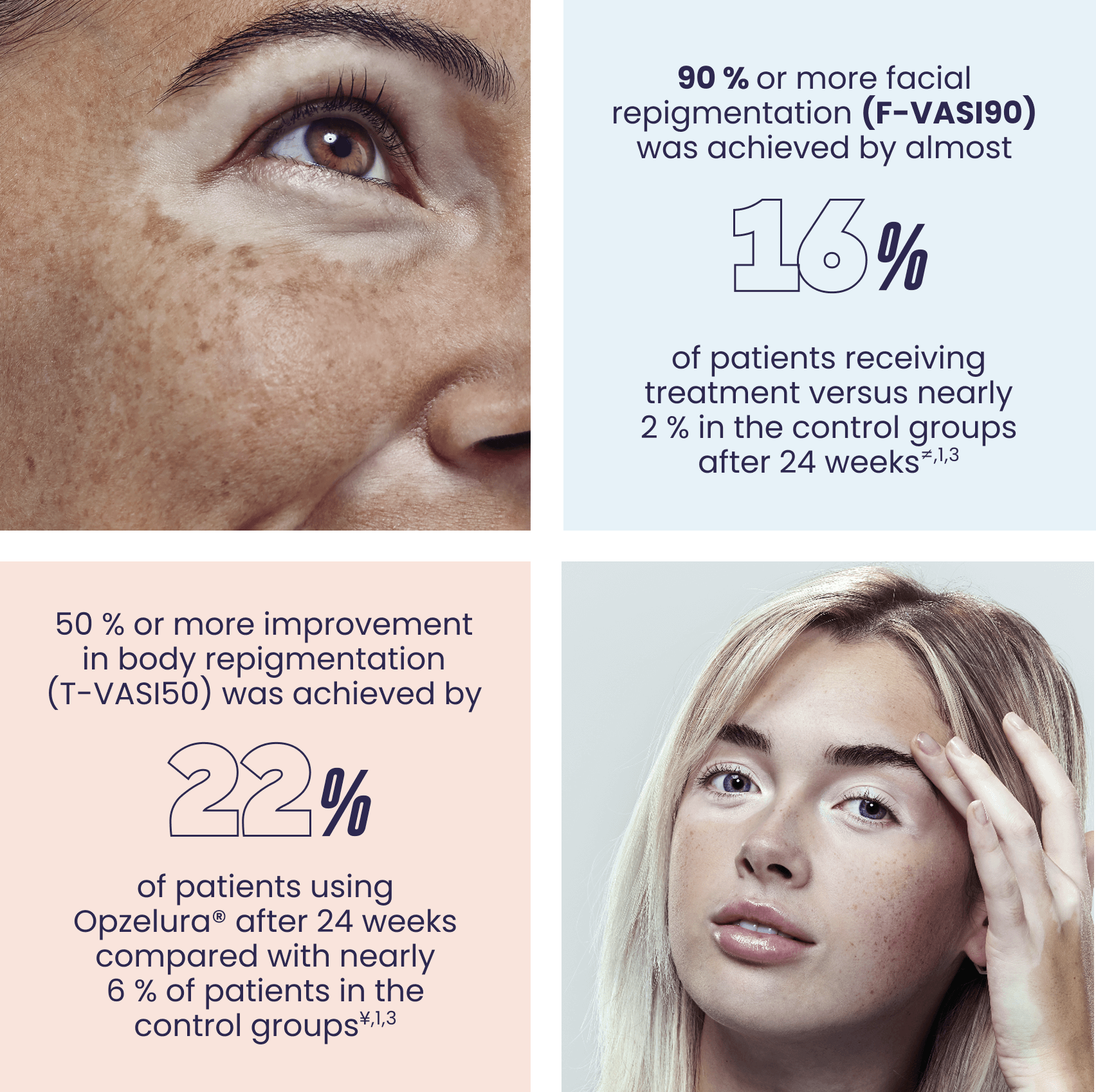
≠The percentages are the average of the secondary endpoint (F-VASI90) from the TRuE-V1 and TRuE-V2 pivotal studies as referenced in Table 2 of the SPC. TRuE-V1: N=221/330 patients; Response rate difference between test and control (95% CI) = 13.2 (7.497 to 18.839) p<0.005. TruE-V2: N= 222/331 patients; Response rate difference between test and control (95% CI) = 15.0 (9.250 to 20.702) p<0.01.1,3
¥The percentages are the average of the secondary endpoints (T-VASI50) from the TRuE-V1 and TRuE-V2 pivotal studies as referenced in Table 2 of the SPC. TRuE-V1: N=221/330 patients; Response rate difference between test and control (95% CI) = 15.5 (8.339 to 22.592) p<0.005. TruE-V2: N= 222/331 patients; Response rate difference between test and control (95% CI) = 17.1 (9.538 to 24.721) p<0.001.1,3
Prescribing information
Opzelura (ruxolitinib) 15 mg/g kräm. Rx. EF. ATC kod: D11AH09, medel vid dermatit, exkl. kortikosteroider. Indikation: Opzelura är avsett för behandling av icke-segmentell vitiligo med ansiktsengagemang hos vuxna och ungdomar från 12 års ålder. Kontraindikationer: Överkänslighet mot den aktiva substansen eller mot något hjälpämne. Graviditet och amning. Varningar och försiktighet: Krämen är inte avsedd för okulär, oral eller intravaginal användning. Om krämen oavsiktligt skulle komma i ögonen eller på slemhinnor ska den torkas av noga och/eller sköljas bort med vatten. Ickemelanom hudcancer (NMSC), främst basalcellskarcinom, har rapporterats hos patienter som behandlats med topikalt ruxolitinib. Något orsakssamband med topikalt ruxolitinib har inte fastställts. Undersökning av huden med jämna mellanrum rekommenderas för alla patienter, särskilt dem med riskfaktorer för hudcancer. Innehåller hjälpämnen som kan ge hudirritation, lokala hudreaktioner, allergiska reaktioner eller vara irriterande för ögon och slemhinnor. Graviditet och amning: Fertila kvinnor ska använda effektiv preventivmetod under behandlingen och i minst 4 veckor efter avslutad behandling. Opzelura är kontraindicerat under graviditet och amning. Behandlingen måste avbrytas cirka 4 veckor innan amning påbörjas. MAH: Incyte Biosciences Distribution B.V., Paasheuvelweg 25, 1105 BP Amsterdam, Nederländerna. Kontakt: Incyte Biosciences Nordic AB, Barnhusgatan 3, 111 23, Stockholm. Datum för översyn av produktresumén: 2025-07-03. För ytterligare information samt pris vänligen se www.fass.se.
Contact
Incyte BiosciencesNordic AB
Barnhusgatan 3
111 23 Stockholm Sweden
+46 8 511 615 15


